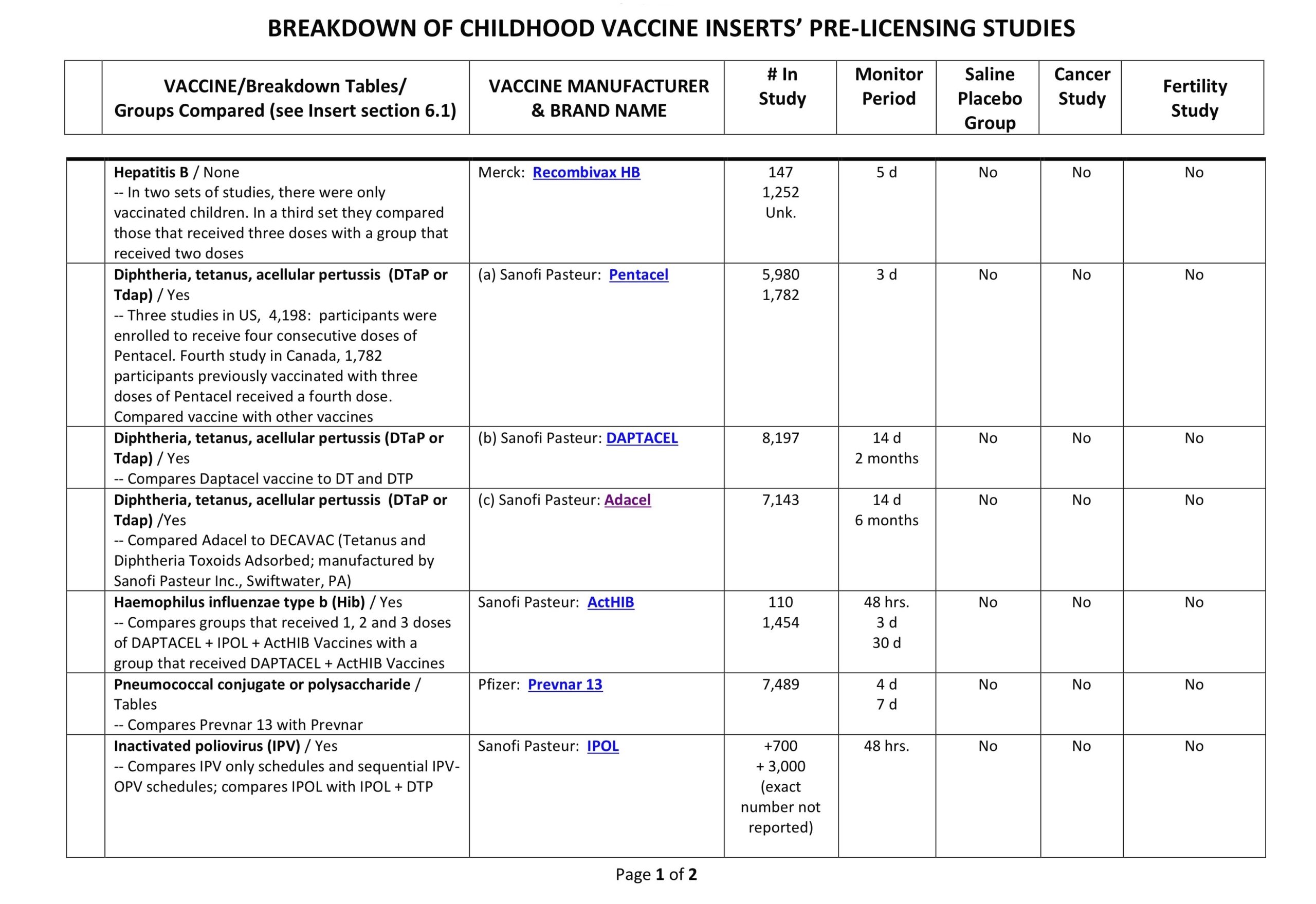
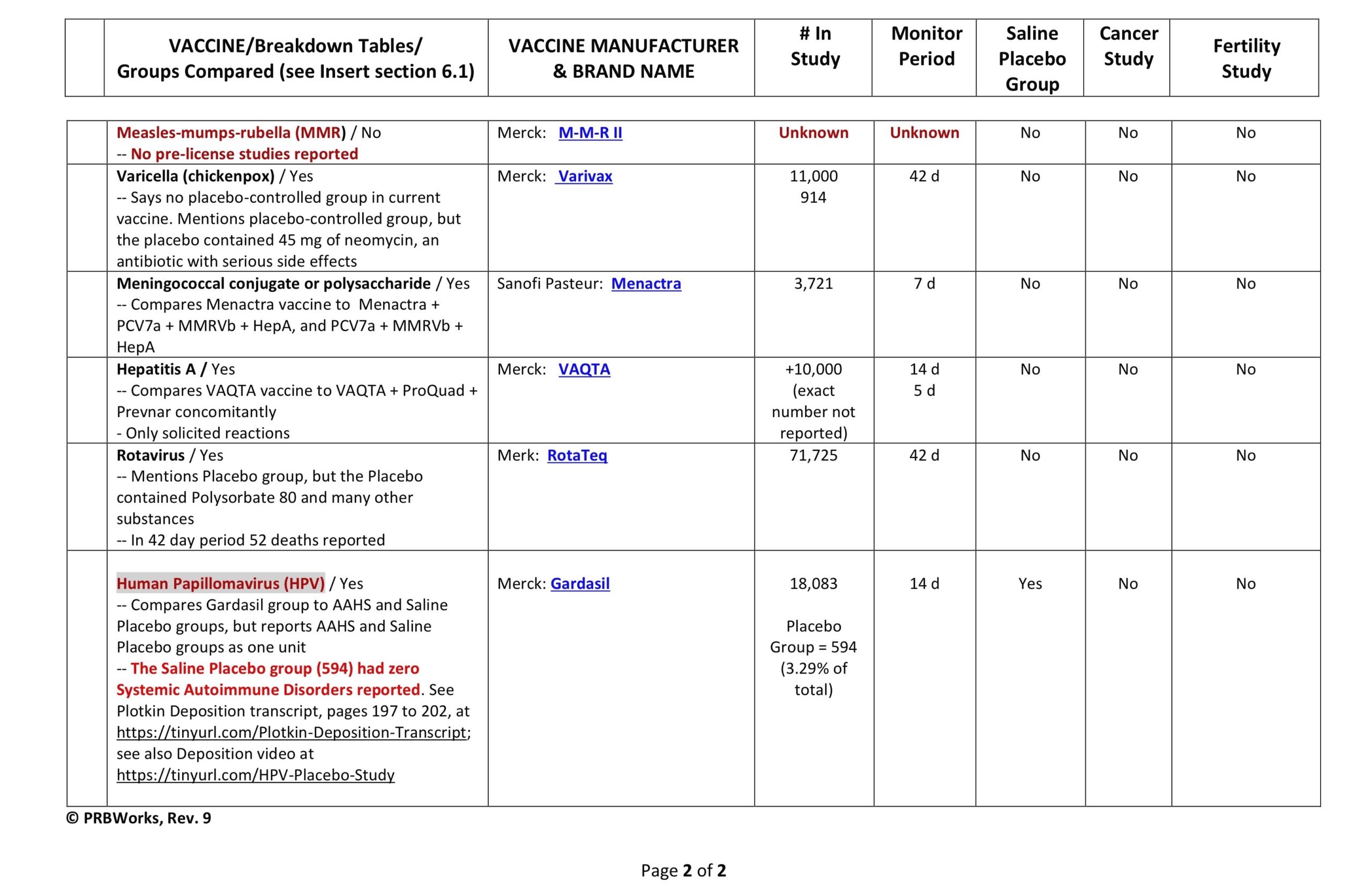
Clinical Trial Safety-Testing Duration
It is often stated in the media and by public health officials that vaccine products are tested for “years” before coming to market. Is that true? Yes and no. Sometimes efficacy studies go on for years, but safety studies–according to all the data provided to the FDA by vaccine manufacturers and which is required by law to be included on vaccine inserts–can be as short as days.
Yet the CDC states:
“Are vaccines safe for children?
Yes. The United States has the safest, most effective vaccine supply in its history. Years of testing are required by law to ensure that vaccines are safe before they are made available in the United States. This process can take 10 years or longer.”
Below is a table showing the duration of the safety arms of clinical trials for a few vaccine products currently on the market. This is the data the FDA based their decisions on when licensing a vaccine product. You be the judge. Were the trials large enough, and long enough, to determine safety?
(Our thanks to Ricardo Beas for the table)