September 2017: CDC admits it knew about a safety signal in 2015 for the flu shot during pregnancy.
On September 13, 2017, the CDC posted a new web page – Flu Vaccination & Possible Safety Signal – in response to their own study that showed that the overall risk of SAB (spontaneous abortion/miscarriage) was twice as high for women who had received the flu vaccine during pregnancy in the 28 days following vaccination compared to those who had not and 7.7 times as high if the woman had received an H1N1-containing vaccine in the previous season. This was a small, case-control study that took place over two flu seasons. It was not designed to estimate the risk of miscarriage following vaccination during pregnancy, but the CDC acknowledges the results to be a red flag.
In response to the sudden spotlight on the study, the CDC created a web page that states:
A Potential Safety Signal Associated with Flu Vaccination of Pregnant Women
A CDC-funded study found that women vaccinated early in pregnancy with a flu vaccine containing the pandemic H1N1 (H1N1pdm09) component and who also had been vaccinated the prior season with a H1N1pdm09-containing flu vaccine had an increased risk of spontaneous abortion (miscarriage) in the 28 days after vaccination. While most miscarriages occurred in the first trimester, several occurred during the second trimester. The median gestational age at the time of miscarriage was 7 weeks. This study does not quantify the risk of miscarriage and does not prove that flu vaccine was the cause of the miscarriage. Earlier studies have not found a link between flu vaccination and miscarriage. There is an ongoing investigation to study this issue further among women who were pregnant and eligible to receive flu vaccine during the 2012-13 through 2014-15 flu seasons. Results are anticipated in late 2018 or 2019.
CDC and its Advisory Committee on Immunization Practices (ACIP) are aware of these data, which were first presented to ACIP at a public meeting in June 2015. At this time, CDC and ACIP have not changed the recommendation for influenza vaccination of pregnant women.
InformedChoiceWA is highly concerned it took two years and this spotlight for the CDC to alert the public to this safety signal.
This delay is yet another example of how the CDC has failed to put the best interests of the public and individuals ahead of their vaccination goals. In this Regulatory Vacuum, individuals are on their own to learn all the information they need to know to make informed vaccination decisions. InformedChoiceWA is here to provide as much information as possible. One of the most critical facts is this, and we are saying it boldly because of its importance:
ALERT: If your doctor has told you that you NEED to get a vaccine during pregnancy to protect your baby and that vaccination during pregnancy has been proven to be safe and effective, your right to fully informed medical consent has been violated.
We have been alerted that some clinics are telling pregnant woman who decline a flu OR TDAP shot that they must sign a form which states they are endangering themselves and others. You are under no obligation to sign the form and asking you to do so is a violation of your human right to fully informed consent for yourself and the unborn child.
Please send the name of any clinic, doctor, or vaccine provider who is using such forms to contact @ informedchoicewa.org
While the CDC has been recommending pregnant women get both a flu and Tdap shot for years, until 2022, that recommendation was “off label” and women were not told. FDA had stated:
“Several licensed vaccines may be used during pregnancy to prevent disease in the mother, unless specifically contraindicated, Tdap & Influenza. Vaccines recommended for pregnant women were first licensed and approved for use based on safety and effectiveness data in non-pregnant women. These vaccines were then recommended by public health policy makers for pregnant women based on their perceived benefit and minimal risk for the mother and infant. Currently, no vaccine is approved specifically for use during pregnancy to protect the infant.“
Currently, no flu vaccines are licensed for use in pregnancy for protection of the infant.
Tdap vaccines have been licensed for this use. On October 7, 2022 the FDA licensed the Tdap brand Boostrix for use in pregnancy for protection of infants younger than 2 months of age. However, the clinical review documents are a tangle of re-worked studies, studies of non-U.S. product formulations, studies that excluded non-live birth outcomes, and no longterm safety studies. There are no vaccinated vs fully non-vaccinated studies, meaning, no control groups of infants who received no vaccines in utero or after birth. See the review document: https://www.fda.gov/media/162830/download
And see this critical review: Maternal Gestational Tdap Vaccination and Autism: A Critique of Becerra-Culqui et al. (2018)
This is the FDA’s slide showing the stages of clinical development that would lead to licensure of vaccines for use during pregnancy to protect the infant. All pregnant women who have been given a vaccine are–in essence–in the Pregnant Women – Phase 3 group without their knowledge or consent. They are being told the vaccines are being given to them to protect the infant–but safety and protection for the fetus/infant have not been established and the vaccines have not been licensed for that purpose.
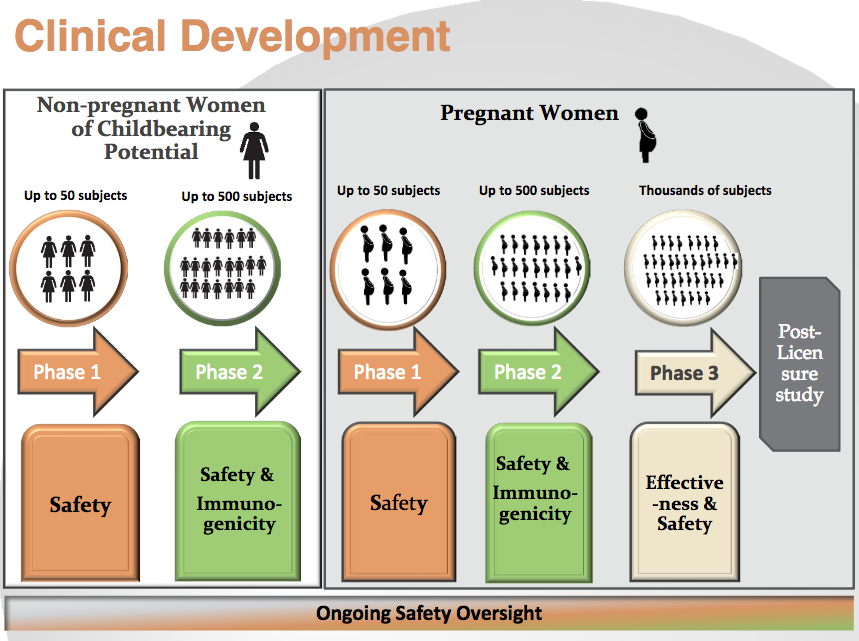
The majority of women vaccinated during pregnancy are not tracked and data on outcomes is not gathered.
- VAERS (Vaccine Adverse Event Reporting System) Few adverse events are voluntarily reported to VAERS (estimated at 1-10%)
- Drug companies have set up voluntary registries; they report to the FDA. There is no public access to the data.
- (VSD), the Vaccine Safety Datalink (VSD) is a collaborative project between the CDC and 9 integrated health care organizations. The VSD includes data on approximately 3% of the US population. Access by independent researchers extremely limited.
- Only a small fraction of the outcomes of vaccinated-pregnancies are known.
Several of the studies the CDC cites as showing safety excluded pregnant women who experienced spontaneous abortion; some only looked at pregnancies that resulted in live birth. There are no reliable long-term outcome studies available to show if receipt of the Tdap, which contains 250mcg of aluminum, capable of crossing the placenta and blood-brain barrier, or receipt of the flu vaccine (some of which contain thimerosal), impacts neurological or immune system development.
It has been established, however, that activation of the maternal immune system can negatively impact the fetus and has been linked with autism. Only a small percentage of women catch pertussis or influenza during pregnancy. But maternal vaccination programs ensure that ALL pregnant women experience immune activation, thus increasing the number of infants put at risk.
The TdaP contains 250 mcg of aluminum, a known neurotoxin capable of crossing the placenta. Comparisons with ingested aluminum are not appropriate. Only .3% of aluminum that is ingested is absorbed while 100% of injected aluminum is absorbed. Please see our Aluminum page for more information.
In 2004, the ACIP began recommending pregnant women get a flu shot in every pregnancy regardless of the trimester. They based this decision on just two studies which showed neither necessity nor safety. A critical paper by David M. Ayoub, M.D. and F. Edward Yazbak, M.D on this decision can be found HERE.
In 2012, the ACIP (the CDCs Advisory Committee on Immunization Practices) began recommending that pregnant women get a Tdap in every pregnancy, no matter when she had last received a Tdap. This was done because the “p” portion of the vaccine, pertussis, is failing. Please see our Pertussis page for details.
TIMELINE of recommendations. Read the linked MMWRs to see the experimental and presumptive nature of the recommendations.
-
- In 2004, the ACIP began recommending pregnant women get a flu shot in every pregnancy regardless of the trimester. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5306a1.htm
- They based this decision on just two studies which showed neither necessity nor safety. A critical paper by David M. Ayoub, M.D. and F. Edward Yazbak, M.D on this decision can be found at http://www.jpands.org/vol11no2/ayoub.pdf
- 2008 Guiding Principles for Development of ACIP recommendations for Vaccination during Pregnancy and Breastfeeding https://www.cdc.gov/vaccines/acip/committee/downloads/preg-principles-2008.pdf
- 2011 (October) Tdap added for those who previously have not received a Tdap. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6041a4.htm
- 2012 (July) Tdap recommended to pregnant women in an MMWR. https://www.cdc.gov/mmwr/pdf/wk/mm6128.pdf
- 2012 (October) ACIP voted to recommend during every pregnancy, regardless of vaccination status. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6207a4.htm
Risk vs Benefit?
For the flaws, limitations, and general risks (such as increasing risk of non-vaccine infections) of the vaccines currently given to pregnant women, please see our Pertussis Page and Flu page. As this page reveals, the risks of the vaccines to unborn babies, and the impact on their immune and neurological development is not known.
The CDC says, “Flu is more likely to cause severe illness in pregnant women than in women who are not pregnant.” We again refer you to the Ayoub & Yazbak paper, and Dr. Jennifer Margulis’s article. On average, just 5 pregnant women die from flu complications in non-pandemic years. In pandemic years, the rates are higher. During the 2009-2010 pandemic, 75 pregnant women died. The data does not reveal their vaccination status, but a study reveals those who receive an annual vaccination may be at more risk of catching pandemic flu. The pandemic was caused by a new flu strain: H1N1pdm09. This strain was included in the vaccine the next season, and it was receipt of this vaccine that increased risk of miscarriage 7.7 fold the following year.
For pertussis, the CDC says “Most of the deaths each year are in babies younger than 3 months of age.” How many? In 2012, during an epidemic of pertussis in which more than 48,000 people in the US were diagnosed, 15 infants died. Provisional data from 2016 shows 6 deaths under age 1.
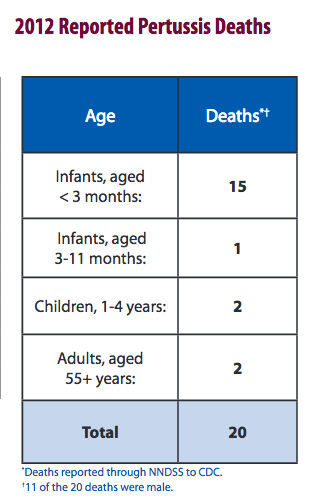
Newborn pertussis risk is extremely small. Baxter et al., 2017 report the improvement in relative risk among babies of vaccinated mothers. However, the data from Baxter et al. provide an absolute risk reduction (ARR) in infants of just 0.02%, i.e., 15 pertussis cases out of 79,292 unvaccinated mothers vs. 1 case out of 68,168 mothers vaccinated at least 8 days prior to giving birth. Baxter et al. limited the birth mothers to those born prior to 1996 for the purpose of ensuring all mothers had received the whole-cell pertussis primary series, so even this small ARR may not be afforded to infants born to women who received acellular vaccines as their primary series.
So while both flu and pertussis during pregnancy can be potentially dangerous to pregnant women and their babies, the risk of acquiring either infection is very low, and the risk of death is extremely low. Based on a birth rate of around 4 million babies a year in the US, the crude figures are:
- Risk of flu-complication death for pregnant women: .000125%
- Risk of flu-complication death for pregnant women in a pandemic year: .001875%
- Risk of newborn death under three months during a non-major outbreak year: .00015%
- Risk of newborn death under three months during a major whooping cough outbreak: .000375%
Anything that triggers immune activation during pregnancy–including flu and pertussis, and including vaccination which is designed to provoke an immune response–can lead to complications and harm to the developing fetus. The odds of getting the flu or pertussis while pregnant and thus activating the immune system are low. Being vaccinated during pregnancy guarantees immune activation 100% of the time.
Knowledge erases fear and allows fully informed decisions. Maintaining a healthy diet and lifestyle before, during, and after pregnancy are essential for an uncomplicated pregnancy and the health of the newborn. See a trusted professional about how best to prepare for pregnancy, or safe ways to avoid infection while pregnant, and how to best protect your newborn. You can empower yourself with no-risk natural and common sense options.
* * * * *
Flu Vaccine:
“Safety and effectiveness of FLUVIRIN® have not been established in pregnant women, nursing mothers or children less than 4 years of age.”
“Pregnancy Category B: A reproductive and developmental toxicity study has been performed in rabbits at a dose level that was approximately 15 times the human dose based on body weight. The study revealed no evidence of impaired fertility or harm to the fetus due to FLUVIRIN®. There are, however, no adequate and well-controlled studies in pregnant women.”
“Nursing Mothers: It is not known whether FLUVIRIN® is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when FLUVIRIN® is administered to a nursing woman.”
“The 0.5-mL prefilled syringe presentation is formulated without preservative. However, thimerosal, a mercury derivative used during manufacturing, is removed by subsequent purification steps to a trace amount (≤ 1 mcg mercury per 0.5-mL dose). The 5-mL multidose vial formulation contains thimerosal, a mercury derivative, added as a preservative. Each 0.5-mL dose from the multidose vial contains 25 mcg mercury.”
http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM123694.pdf
CDC-cited STUDIES
The following studies are cited by the CDC (also HERE) as showing safety for use in pregnancy. Note that, unless it was a randomized clinical trial (of which there are very few) the subjects were not knowingly enrolled in safety studies. They were pregnant women who were told by their doctors, who trust the CDC to guide them, that the vaccines were safe during pregnancy and effective for protecting the baby. We are reviewing the studies to see if they specifically address safety for the fetus, in particular, do they address non–live birth outcomes (stillborn, spontaneous abortion/miscarriage) and/or long-term impacts on the immunological, developmental, or neurological health of the child.
Below are guidelines for determining the Level of Evidence for papers:
Do the studies the CDC cite as showing safety of vaccination in pregnancy address non–live birth outcomes (stillborn, spontaneous abortion/miscarriage) and/or long-term impacts on the immunological, developmental, or neurological health of the child?
Study 1: No
Study: Sukumaran L, McCarthy NL, Kharbanda EO, et al. Association of Tdap vaccination with acute events and adverse birth outcomes among pregnant women with prior tetanus-containing immunizations. JAMA. 2015;314(15):1581–7. PMID: 26501534
Type: Retrospective cohort (Level 3); VSD Data
Conclusion: “There were no statistically significant differences in rates of medically attended acute adverse events or adverse birth outcomes related to timing since prior tetanus-containing vaccination.”
Study Objective: “To determine whether receipt of Tdap vaccine during pregnancy administered in close intervals from prior tetanus-containing vaccinations is associated with acute adverse events in mothers and adverse birth outcomes in neonates.”
Excluded: “We excluded women who had no documentation of prior tetanus-containing vaccines, women who received live vaccines during pregnancy, and women with a multiple gestation pregnancy. We also excluded pregnancies with non–live birth outcomes (stillborn, spontaneous abortion, therapeutic abortion, trophoblastic disease, and ectopic pregnancy) because we did not have the resources to access medical records to confirm the timing of these outcomes in relation to vaccination, which could result in inaccurate findings. Finally, we excluded all women who received non-Tdap tetanus-containing vaccines during pregnancy (ie, tetanus diphtheria [Td]).”
Weakness/Flaw: Exclusion of all non-live birth outcomes makes the conclusion of this study extremely misleading. It does not speak at all to the safety of vaccination during pregnancy since rates of spontaneous abortion or fetal demise were not considered; current ACIP recommendations would lead to much higher frequency TdaP administration than this study examined; long-term health outcomes of children were not examined; rates of adverse reactions were not compared with a non-vaccinated control group.
Study 2: No
Study: DeSilva M, Vazquez-Benitez G, Nordin JD, et al. Maternal Tdap vaccination and risk of infant morbidity. Vaccine. 2017;35(29):3655–60. PMID: 28552511
Type: Retrospective cohort (Level 3); VSD Data
Excluded: All non-live birth outcomes
Study Conclusion: “Despite an observed association between maternal Tdap vaccination and maternal chorioamnionitis, we did not find increased risk for clinically significant infant outcomes associated with maternal chorioamnionitis.”
Weakness/Flaw: By excluding non-live birth outcomes and by not looking at all newborn or long-term health complications, this study provides no insight into the potential risk of maternal vaccination.
About chorioamnionitis: “Chorioamnionitis is a common complication of pregnancy associated with significant maternal, perinatal, and long-term adverse outcomes. Adverse maternal outcomes include postpartum infections and sepsis while adverse infant outcomes include stillbirth, premature birth, neonatal sepsis, chronic lung disease and brain injury leading to cerebral palsy and other neurodevelopmental disabilities. Research in the last two decades has expanded our understanding of the mechanistic links between intraamniotic infection and preterm delivery as well as morbidities of preterm and term infants.” See Diagnosis and Management of Clinical Chorioamnionitis. PMCID: PMC3008318
Study 3: meta review
Study: McMillan M, Clarke M, Parrella A, Fell DB, Amirthalingam G, Marshall HS. Safety of tetanus, diphtheria, and pertussis vaccination during pregnancy: A systematic review. Obstet Gynecol. 2017;129(3):560–73. PMID:28178054
Type: Meta-Review
The authors stated this:
“Although there are compelling reasons to institute population-wide administration of antenatal pertussis containing vaccines, there is limited information about the safety of antenatal vaccination (and revaccination) for the mother, fetus, and newborn. An inflammatory response from infection during pregnancy has been shown to increase the risk of fetal injury. Despite no evidence that an inflammatory response from an inactivated vaccine carries a similar risk, it is biologically plausible.”
Weakness/Flaw: The review states: “There was substantial clinical and methodologic heterogeneity from mainly retrospective observational studies with an overall high risk of bias. ” When we have examined the 21 studies examined in this review, we will provide an update.
Study 4: No
Study: DeSilva M, Vazquez-Benitez G, Nordin JD, et al. Tdap vaccination during pregnancy and microcephaly and other structural birth defects in offspring. JAMA. 2016;316(17):1823–5. PMID: 27802536
Type: Retrospective cohort (Level 3), VSD data
Excluded: “Insurance and health utilization criteria were not applied for infant deaths at less than 1 year. Infants with exposures increasing risk for structural birth defects (maternal diabetes or use of teratogenic medications, infant congenital infections, and chromosomal abnormalities) were excluded.”
Study Conclusion: “These results expand upon what is known about maternal Tdap vaccination safety6 to include information about structural birth defects and microcephaly in offspring. The findings are potentially limited by incomplete data on Tdap vaccinations (making it possible to misclassify women’s immunization status), diagnosed structural birth defects, and important covariates (including maternal alcohol use), as well as inability to study birth defects resulting in pregnancy loss or elective termination. The findings support recommendations for routine Tdap administration during pregnancy.”
Weaknesses/Flaws: See bolded sections of the conclusion. Also, mothers with maternal diabetes are routinely vaccinated. The impact of Tdap may be additive to risk, and this group should not have been excluded but examined separately in order to determine maternal groups more susceptible to fetal vaccine adverse effects. All vaccination comes with “non-specific effects” — effects that are observed to occur but were not intended by the developers. Non-specific effects of Tdap includes susceptibility to other infections, especially in the immunocompromised and pregnancy is a state of suppressed immune function (see the video on herd immunity on our Pertussis page for more about non-specific effects.) Tdap may increase risk of viral infection during pregnancy–symptomatic or asymptomatic–leading to congenital infections, so that group should not have been excluded. And studies have shown that exposure to aluminum can induce chromosomal abnormalities (see PMID: 19274764 and our Aluminum page), so that group also should not have been excluded.
Study 5: No
Study: Sukumaran L, McCarthy NL, Kharbanda EO, et al. Safety of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis and influenza vaccinations in pregnancy. Obstet Gynecol. 2015;126(5):1069–74. PMID:26444109
Type: Retrospective cohort (Level 3), VSD data
Excluded: “We excluded women who received any live vaccines in pregnancy, those with multiple gestations, and those with non-live birth outcomes, including stillborn, spontaneous abortion, therapeutic abortion, trophoblastic disease, and ectopic pregnancy, since we did not access medical records to confirm these outcomes and their onset dates. Additionally, we excluded women who received more than one tetanus containing vaccine (including multiple Tdap vaccines) in the same pregnancy and women who received more than one influenza vaccine (seasonal influenza and H1N1 influenza or multiple seasonal influenza vaccines) on different days in the same pregnancy, in order to limit our comparisons to women with a single influenza vaccination date and a single tetanus vaccination date.
Study Conclusion: “Concomitant administration of Tdap and influenza vaccines during pregnancy was not associated with a higher risk of medically attended adverse acute outcomes or birth outcomes compared to sequential vaccination.”
Weaknesses/Flaws: The exclusion of all non-live birth outcomes makes the conclusion of this study irrelevant to informing on the safety of vaccination during pregnancy. The study design compared adverse reactions/outcomes following concomitant vaccination to reactions/outcomes of those receiving the vaccinations at a separate time. For this outcome to be meaningful, besides including non-live birth outcomes, non-vaccinated comparison group data should have been provided. Long-term impacts on the health of the children born live were not examined.
The study points out: “We performed a priori power calculations and determined that we had 80% or higher power to detect relative risks of greater than 2 for all of our birth outcomes, even with the restricted cohort. However, our analyses for medically attended acute outcomes, which are rare, were underpowered. For this reason, we limited our analysis of acute outcomes to fever and any acute event (37,000 and 10,000 pregnancies needed in each cohort respectively to detect a relative risk of 2). We considered results to be statistically significant at an alpha error less than 0.05 using 2-tailed tests.”
Study 6: Yes for pregnancy loss (flawed design–limited by high-risk comparison group); No for long-term outcomes
Study: Morgan JL, Baggari SR, McIntire DD, Sheffield JS. Pregnancy outcomes after antepartum tetanus, diphtheria, and acellular pertussis vaccination. Obstet Gynecol. 2015;125(6):1433–8. PMID: 26000515
Type: Retrospective cohort (Level 3), single medical institution
Conclusion: “No adverse pregnancy outcomes were identified in association with antepartum Tdap vaccination. This remained true in women receiving more than one Tdap vaccine in a 5-year timeframe. This may be the result of a type II error.”
This passage from the paper provides insight into the issue we are presenting here: it explains that despite CDC recommendations, safety has not been established.
Weaknesses/Flaws: The authors state their study is limited by the possibility of “type II” error because of their fixed sample size, especially in regard to rare study outcomes such as stillbirth and neonatal death. They state there was a potential for unmeasured cofounders, and they did not have access to relevant historical information or any data about vaccinations, adverse outcomes, or births that did not happen at the study institution. The vaccinated subjects numbered 97% of the total, and the comparison non-vaccinated just 3%, and the authors state this group was likely may at increased risk of preterm birth or other complications–this is reflected in the 92% that were referred to high-risk obstetrics compared to 70% of the vaccinated group. This healthy-user bias makes it impossible for any useful conclusions to be drawn from this study.
Study 7: No
Study: Czeizel AE, Rockenbauer M. Tetanus toxoid and congenital abnormalities. Int J Gynecol Obstet. 1999;64:253–8. PMID: 10366047
Type: Hungarian Case–Control Surveillance (Level 3)
Conclusion: “Tetanus vaccination during pregnancy appears not be teratogenic to the fetus. Thus, there is no contraindication, if the use of tetanus toxoid is necessary during pregnancy.”
Weaknesses/Flaws: The study covered periods when maternal vaccination was rare. “Of 35 727 pregnant women who had babies without any defects in the study period (control group), 33 (0.09%) were vaccinated with tetanus. Of 21 563 pregnant women who had offspring with congenital abnormalities, 25 (0.12%) had tetanus vaccination. This difference was not significant (P=0.39).” Drawing the conclusion that use of a tetanus toxoid during pregnancy is non-tetragenic based on such limited subjects is irresponsible.
Study 8: Yes for pregnancy loss (study design unable to detect risk under twofold); No for long-term outcomes
Study: Donegan K, King B, Bryan P. Safety of pertussis vaccination in pregnant women in UK: Observational study. BMJ. 2014;349:g4219. PMID 25015137.
Type: Observational cohort (Level 3)
Study Conclusion: “Our results showed no significantly increased risks, and, indeed, confidence intervals are such that in general we can exclude twofold risks. The analysis presented, however, cannot rule out smaller increases in risk, and the short study period limits the possibility of examining longer term adverse events. The vaccination programme remains ongoing, and the MHRA continues to monitor the safety of the vaccine in pregnancy with the aim of identifying any smaller increases in the risk of adverse events related to pregnancy as well as longer term safety.”
Study 9: No
Study: “Adverse events in pregnant women following administration of trivalent inactivated influenza vaccine and live attenuated influenza vaccine in the Vaccine Adverse Event Reporting System, 1990-2009.” American Journal of Obstetrics and Gynecology, Mosby, 20 Oct. 2010, www.sciencedirect.com/science/article/pii/S0002937810011051. PMID:20965490
Type: Survey of VAERS
Study Conclusion: “No unusual patterns of pregnancy complications or fetal outcomes were observed in the VAERS reports of pregnant women after the administration of TIV or LAIV.”
Weaknesses/flaws: The voluntary and passive VAERS suffers from critical under-reporting. While surveys of the data can sometimes indicate the need for further research, no study using only VAERS data can ever be conclusive or show safety.
Study 10: under review
Study: Irving, S A, et al. “Trivalent inactivated influenza vaccine and spontaneous abortion.” Obstetrics and gynecology., U.S. National Library of Medicine, Jan. 2013, www.ncbi.nlm.nih.gov/pubmed/23262941. PMID:23262941
Type: Retrospective case-control (Reported Level of Evidence: 2), VSD data
Conclusion: “There was no statistically significant increase in the risk of pregnancy loss in the 4 weeks after seasonal inactivated influenza vaccination.”
Study under review by ICWA.
Initial remarks: This study design appears unable to find meaningful data. A 28 day vaccine-exposure window was examined and compared between women who experienced spontaneous abortion and those who did not (the control group, who were individuals with a live birth.) This comparison is meaningless, revealing nothing about why one woman vaccinated in the window lost her child when another woman didn’t. Exposure to the vaccine can’t be ruled out as the cause. Only questions can be generated by this study, such as, are there genetic and environmental factors unique to each woman that lead pregnancy loss in the 28 day window following vaccination?
Study 11: No
Study: Kharbanda, E O, et al. “Inactivated influenza vaccine during pregnancy and risks for adverse obstetric events.” Obstetrics and gynecology., U.S. National Library of Medicine, Sept. 2013, www.ncbi.nlm.nih.gov/pubmed/23921876.
Type: Retrospective case-control (Reported Level of Evidence: 2), VSD data
Conclusion: “In this large cohort, influenza vaccination during pregnancy was not associated with increased risks for medically attended adverse obstetric events.”
Study Objective: “To compare risks for adverse obstetric events between females who did and did not receive trivalent inactivated influenza vaccine during pregnancy.” These 13 events were looked for: hyperemesis, chronic hypertension, gestational hypertension, gestational diabetes, proteinuria, urinary tract infection, gestational hypertension, preeclampsia or eclampsia, chorioamnionitis, puerperal infection, venous complications, pulmonary embolism, and peripartum cardiomyopathy.
This study did not include collecting data on spontaneous abortion, fetal demise, stillbirth, or long term health outcomes.
Study 12: No
Study: Nordin, J D, et al. “Maternal influenza vaccine and risks for preterm or small for gestational age birth.” The Journal of pediatrics., U.S. National Library of Medicine, May 2014, www.ncbi.nlm.nih.gov/pubmed/24582484. PMID:24582484
Type: Retrospective observational matched cohort (Level ?), VSD data
Study Conclusion: “Receipt of trivalent inactivated influenza vaccine during pregnancy was not associated with increased or decreased risk of preterm or SGA birth. These findings support the safety of vaccinating pregnant women against influenza during the first, second, and third trimesters, and suggest that a nonspecific protective effect of the influenza vaccine for these outcomes does not exist.”
Study Objective: “To study the impact of influenza vaccine administered to pregnant women during all trimesters on the rates of preterm and small for gestational age (SGA) births, evaluating both increased and decreased risk.”
This study did not include collecting data on spontaneous abortion, fetal demise, stillbirth, or long term health outcomes.
Study 13: No
Study: Kharbanda, E O, et al. “First Trimester Influenza Vaccination and Risks for Major Structural Birth Defects in Offspring.” The Journal of pediatrics., U.S. National Library of Medicine, Aug. 2017, www.ncbi.nlm.nih.gov/pubmed/28550954. PMID:28550954
Type: Retrospective Observational (Level ?), VSD data
Conclusion: “First trimester maternal IIV exposure was not associated with an increased risk for selected major structural birth defects in this large cohort of singleton live births.”
Study Objective: “To examine risks for major structural birth defects in infants after first trimester inactivated influenza vaccine (IIV) exposures.”
This study included only live birth outcomes, flu vaccine administered during first trimester only, control group may have received flu vaccine during 2nd or 3rd trimester, specific birth defects looked for, children followed until age 1 (autism and other developmental disorders not usually assessed until age 2 or later).
Studies not considered by the CDC
See our Aluminum page for studies on the neurotoxicity of aluminum adjuvants. The lack of studies on the impact of aluminum exposure in utero is astounding. The Tdap vaccine contains 250mcg of aluminum capable of crossing the placenta.
Disclaimer


